Electron Configuration Of Silver cloudshareinfo
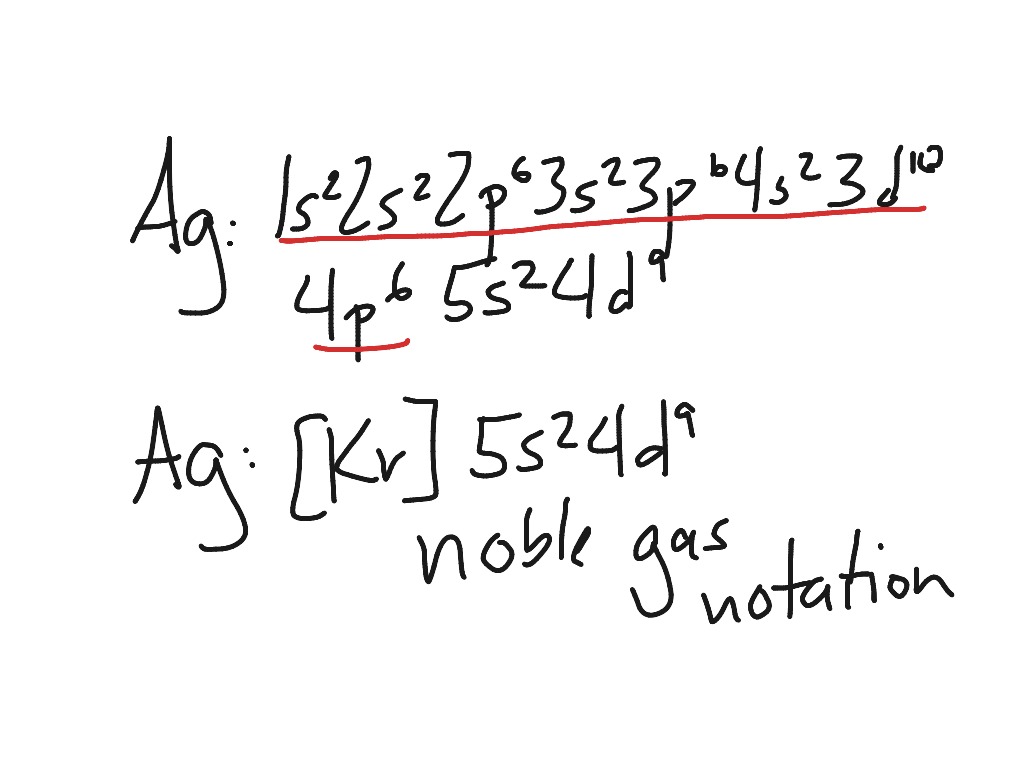
You can get the noble gas electron configuration of any element by following three steps. Let's see how it works-. First, select the input. You can select the element name or atomic number if you want. Enter the element name or atomic number in the blank box based on your input selection. Click the Calculate button.
Noble Gases Electron Configuration
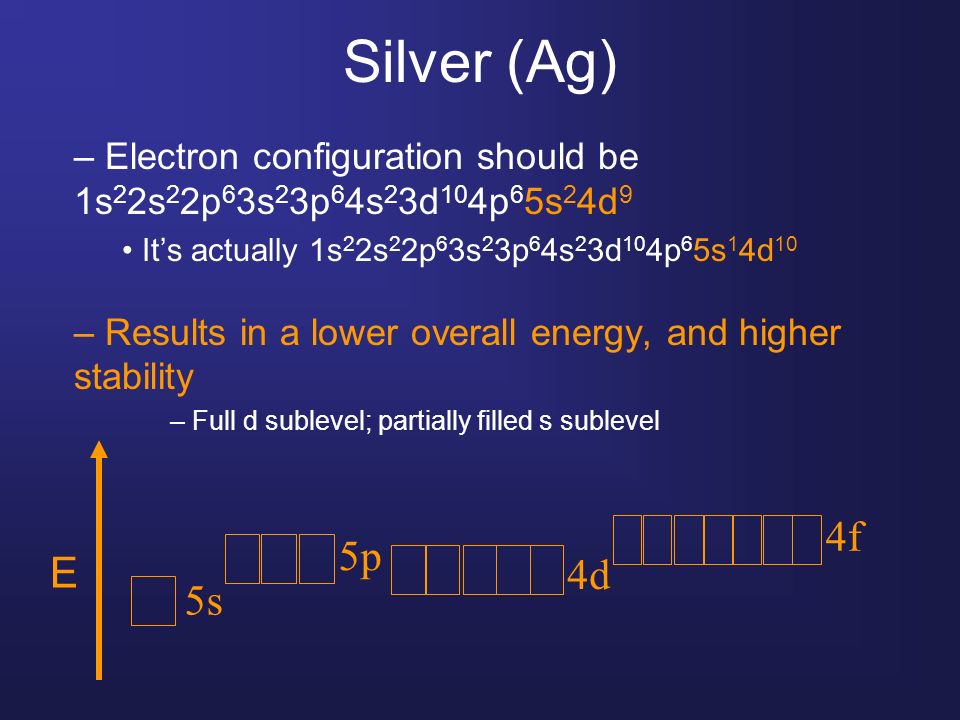
The first 36 electrons are denoted by the noble gas krypton which has 36 as the atomic number. Ag unabbreviated electron configuration.. The electron configuration of silver (Ag), which has an atomic number of 47, is [Kr] 4d^10 5s^1 in the ground state. The unique electron configuration of silver suggests that an electron in the 5s orbital.
Place each noble gas symbol in front of the appropriate partial electron configuration to create

They are helium, neon, argon, krypton, xenon, and radon. A noble gas configuration of an atom consists of the elemental symbol of the last noble gas prior to that atom, followed by the configuration of the remaining electrons. So for sodium, we make the substitution of [Ne] [ Ne] for the 1s22s22p6 1 s 2 2 s 2 2 p 6 part of the configuration.
PPT Chemistry SM1131 Week 14 Lesson 1 PowerPoint Presentation, free download ID5574748

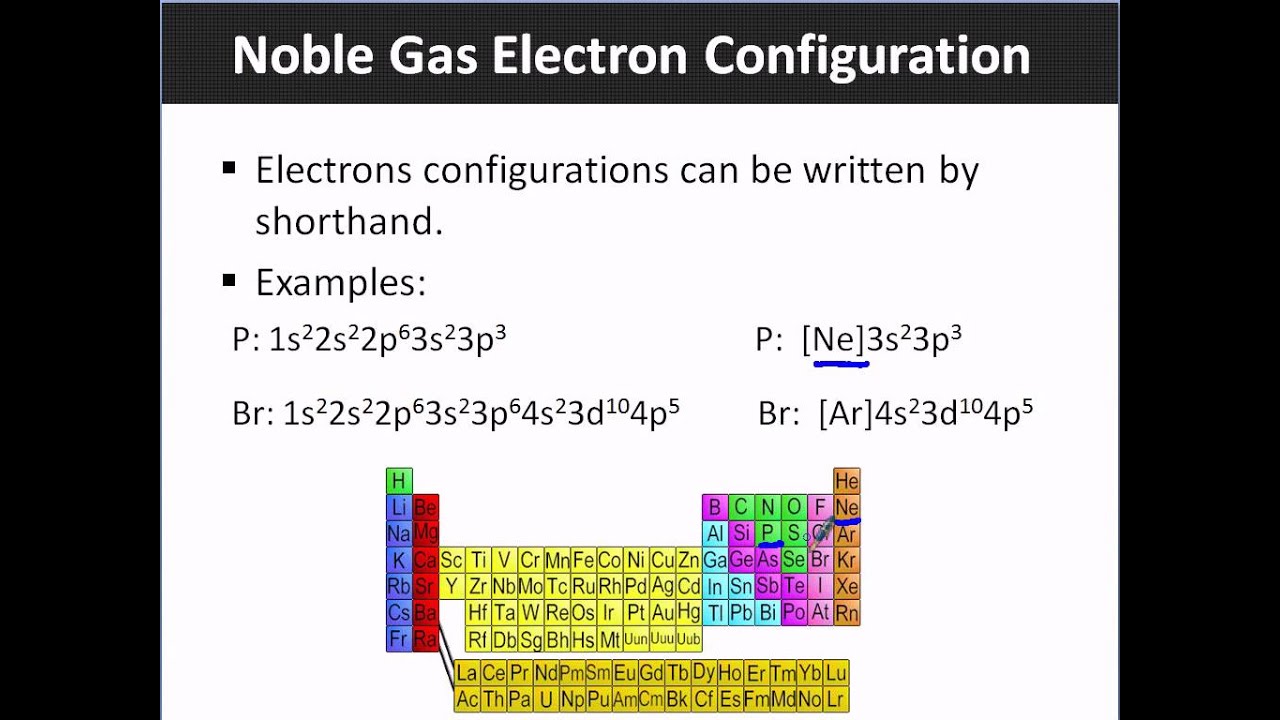
A noble gas configuration of an atom consists of the elemental symbol of the last noble gas prior to that atom, followed by the configuration of the remaining electrons. So for sodium, we make the substitution of [Ne] [ Ne] for the 1s22s22p6 1 s 2 2 s 2 2 p 6 part of the configuration. Sodium's noble gas configuration becomes [Ne] 3s1 [ Ne] 3 s 1.
GChron Technical Note Noble gas extraction procedure and performance of the Cologne Helix MC

The noble gas configuration is written as the elemental symbol of the noble gas in the period before the element followed by the element's remaining electrons. For instance, sodium's full configuration is 1s 2s 2 2 2p 6 3s 1 and neon's is 1s 2s 2 2 2p 6. So, sodium's noble gas configuration is [Ne]3s 1 . Part 1.
How To Write Abbreviated Electron

Electron configuration chart of all Elements is mentioned in the table below.The Shorthand electron configuration (or Noble gas configuration) as well as Full.. Electron configuration of Silver (Ag) [Kr] 4d 10 5s 1: 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 4d 10 5s 1: 2, 8, 18, 18, 1: 48: Electron configuration of Cadmium (Cd)
Noble Gas Electron Configurations YouTube
In practice, chemists simplify the notation by using a bracketed noble gas symbol to represent the configuration of the noble gas from the preceding row because all the orbitals in a noble gas are filled. For example, [Ne]. Silver: Z:47 [Kr] 5s 1 4d 10: Period 6: Period 7: Lanthanum: Z:57 [Xe] 6s 2 5d 1: Actinium: Z:89 [Rn] 7s 2 6d 1: Cerium.
Silver Electron Configuration
To write the configuration for the Silver and the Silver ion, first we need to write the electron configuration for just Silver (Ag). We first need to find.
Noble Gas Configuration Calculator CALCULATOR HGW

A Noble Gas is a group of elements that in their standard state have a filled electron cloud.. These elements are found in the 18th column of the periodic table and include Helium (He), Neon (Ne), Argon (Ar), Krypton (Kr), Xenon (Xe) and Radon (Rn). They are all odourless and colourless mono-atomic elements. Because these elements are already electron stable and do not need to gain or lose.
Noble Gas Configuration Calculator CALCULATOR HGW

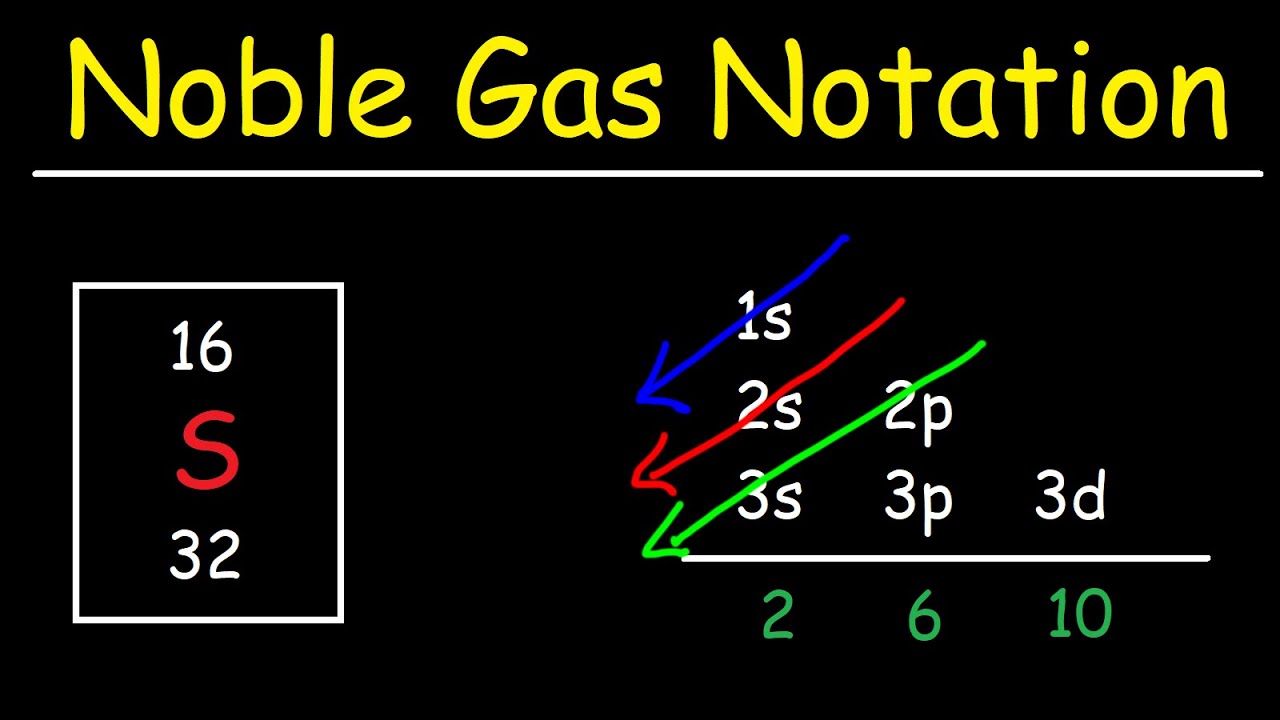
To find the electron configuration of oxygen: Look at the periodic table and find an atomic number of oxygen, which is 8. Fill these 8 electrons in the following order: 1s, 2s, and then 2p. Write the complete electron configuration of oxygen: 1s²2s²2p⁴. Identify the noble gas before oxygen, helium, and write using shorthand notation: [He.
What Is the Full Electron Configuration for Silver AveryhasWall

The electron configuration of silver ion(Ag +) is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 4d 10. This electron configuration shows that the silver ion(Ag +) has four shells and the last shell has eighteen electrons and it achieves a stable electron configuration. Silver atoms exhibit +1 oxidation state.. Krypton is a classified noble gas.
What Is the Noble Gas Configuration for Barium
The electron configuration for silver (Ag) is based upon the place meant of silver in the fifth row of the periodic table in the 11th column of the periodic table or the 9th column of the transition metal or d block. Therefore th electron configuration for silver must end as #4d^9#, #1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^10 4p^6 5s^2 4d^9# This notation can be written in core notation or noble gas.
Chemistry 9/15, 9/18 Noble Gas Configurations, The Atomic Museum Mr. Hollis

So that's the electron configuration for silicon. Now, we can write it out using noble gas notation. And compare, so, the noble gas immediately preceding silicon, if we go up a row and then move over, we see that it's neon. So we write neon in brackets. And then, the other electrons are the ones that come after neon.
What Is the Noble Gas Configuration for Strontium
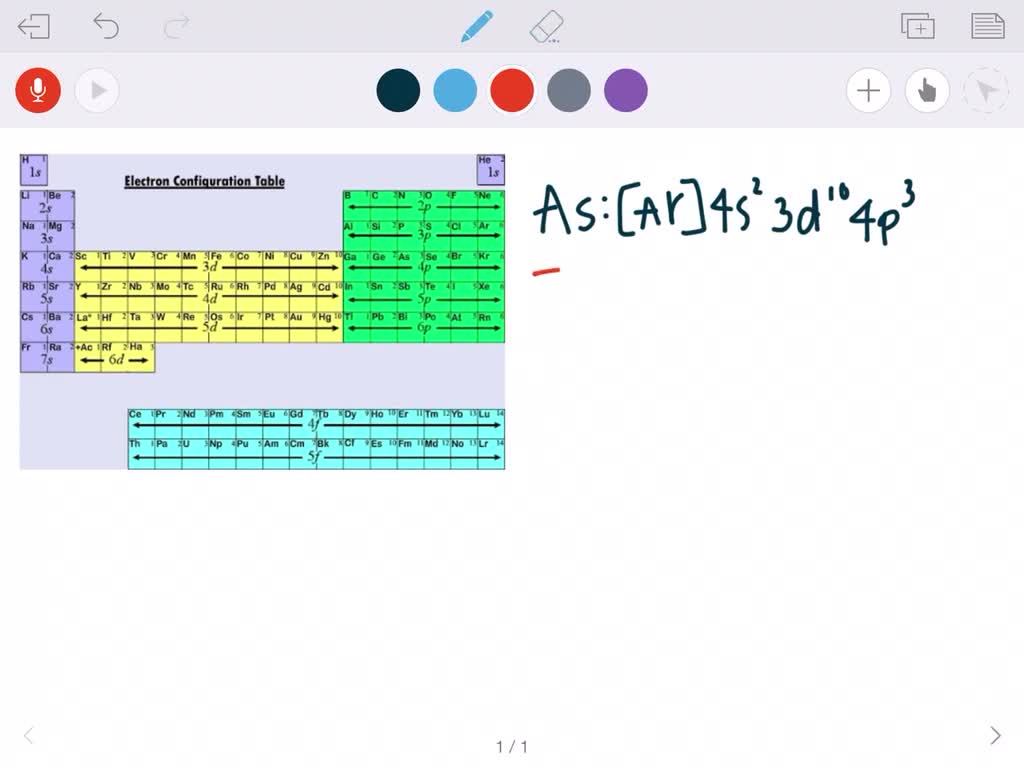
In this case, 2+2+6+2+6+2+10+6+2+1= 39 and Z=39, so the answer is correct. A slightly more complicated example is the electron configuration of bismuth (symbolized Bi, with Z = 83). The periodic table gives the following electron configuration: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p65s2 4d10 5p6 6s2 4f14 5d10 6p3.
Noble Gas Configuration Shorthand Electron Configuration

A silver atom requires seven more valence electrons in its s and p orbital to attain noble gas configuration. What is the complete electron configuration of silver? The complete electron configuration of silver is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 1 4d 10 .
Electron Configuration Of Silver cloudshareinfo

A noble gas configuration of an atom consists of the elemental symbol of the last noble gas prior to that atom, followed by the configuration of the remaining electrons. So for sodium, we make the substitution of [Ne] for the 1 s2 2 s2 2 p6 part of the configuration. Sodium's noble gas configuration becomes [Ne]3 s1.